- Breakthrough Device Designations are given by the U.S. FDA to expedite the review of technologies that can improve the lives of people with life-threatening or debilitating conditions.
- Each year, tens of thousands of Americans suffer from enterocutaneous fistulas (ECF).
- With Breakthrough Device Designation the Limpet™ Device could become available as a new treatment option sooner for these patients.
Fistula Solution, a leading medical device company in complex wound care, announced today that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation to the Limpet™ device for severe abdominal openings unlikely to spontaneously close. This device is designed to reduce current complications associated with enterocutaneous fistulas, which has the potential to reduce healthcare costs and improve patient confidence and quality of life.
Enterocutaneous and other enteric, or intestinal, fistulas are challenging to manage, and the current standard of care is costly. In terms of charges billed to patients, the median total charges per hospital stay are $50,451, with a range of $24,439 to $109,4343. The annual costs of enteric fistulas on the healthcare system are estimated to be close to $500,000,000. Additionally, the charges incurred by enteric fistula patients amount to approximately $1.5 billion. These figures indicate the significant financial burden that enteric fistula patients impose on the healthcare system, highlighting the importance of effective management and prevention strategies for this condition.
The Limpet combines key aspects of fistula management — effluent containment and negative pressure wound therapy for effluent management and promotion of healing of intact and injured skin. The protection of the negative pressure dressing also simplifies nursing care, eliminates the need for frequent dressing changes, and protects the surrounding skin or wound, which all promote the patient’s global health.
“The Breakthrough Device Designation is a significant milestone for our company and validates our belief that the Limpet™ device has advantages over the current standard of care,” said Andy Obst, President of Fistula Solution. “Early clinical results are promising and suggest our device reduces the complications typically associated with fistulas and ostomies and better supports a patient’s ability to get back to life. We look forward to working with the FDA to make the technology accessible as quickly as possible.”
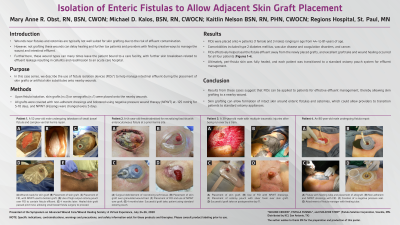
Early clinical data comparing the Limpet with the current standard of care demonstrated that the Limpet achieves a 99.4% average reduction in skin damage area or near total control of intestinal effluent for patients with enteric fistula complications. The Limpet was worn for an average of 3 days versus the 0.26 days for conventional dressings and showed zero leak failures in 14 device placements over 65 days of therapy, reducing patient or caregiver maintenance. Additionally, patients with new skin grafts around their fistula experienced 95% graft take, which is remarkable because grafting around stomas is generally not feasible with conventional dressings.
The FDA Breakthrough Device program is designed to help accelerate the development and approval of medical devices and products that have the potential to provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. Receiving the Breakthrough Device Designation also facilitates the Medicare coverage for the device, which will help the Limpet device become more widely accessible for patients who require an ostomy procedure or develop enteric fistulas, benefitting both the healthcare system and the patient.
About Fistula Solution
Fistula Solution has a portfolio of devices designed to treat enteric fistulas and high output ostomy stomas. The company is committed to developing innovative new devices for post-surgical ostomy complications, rectovaginal fistulas, surgical drain wounds, and necrotizing soft tissue infections.
For further information visit www.fistulasolution.com.
- Moisture-Associated Skin Damage (MASD) | WoundSource
- Taneja, Charu, et al. "Clinical and economic burden of peristomal skin complications in patients with recent ostomies." Journal of Wound, Ostomy, and Continence Nursing 44.4 (2017): 350.
- Brooks NE, Idrees JJ, Steinhagen E, Giglia M, Stein SL. The impact of enteric fistulas on US hospital systems. Am J Surg. 2021;221(1):26-29. doi:10.1016/j.amjsurg.2020.06.017